Use the Pediatric Dosing Calculator for fast, accurate dosing
How to Use
DEFINITY®
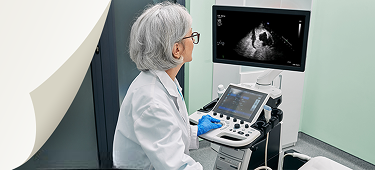

How to Activate DEFINITY®
DEFINITY® is activated using a VIALMIX® RFID, which ensures consistent activation of DEFINITY®. Before activating DEFINITY®, allow the refrigerated vial to warm to room temperature.1,2
DEFINITY® is the only ultrasound enhancing agent that uses mechanical activation, which contributes to its consistent image quality1-4
DEFINITY® can be reactivated, allowing for up to 48 hours of use from the initial activation1,5


How to Store DEFINITY®
DEFINITY® can be conveniently stored in multiple care settings. For greater efficiency, DEFINITY® can be dispensed via a refrigerated solution located within the echo lab for an optimal workflow and convenient sonographer access.1
DEFINITY® is supplied in packages of sixteen 1.5-mL vials.
Store DEFINITY® between 36 °F to 46 °F.
Dosing
DEFINITY® is the only ultrasound enhancing agent with flexible dosing options, including diluted bolus, continuous infusion, and bolus. Each option can be implemented using the PINSYNC® or needle method. Click below for instructions on dosing by your specific method.1,3-5
Select patient type for custom dosing instructions
Step 1 — Choose Administration Method
Step 2 — Choose Withdrawal Technique
Diluted Bolus
DEFINITY® is the only ultrasound enhancing agent approved for use in a diluted form. Diluted bolus is the most common DEFINITY® administration option. This method simplifies administration by combining activated DEFINITY® and preservative-free saline in the same syringe.1,5
How to Prepare DEFINITY® for Diluted Bolus
Diluted Bolus Dosing With PINSYNC® (Vented Vial Adapter 13 mm)8,*
PINSYNC® (Vented Vial Adapter 13 mm) enables the safe, simple, and efficient transfer of activated DEFINITY® from vial to syringe.
Supplies
- PINSYNC®*
- 10-mL syringe filled with 8.7 mL preservative-free saline
Instructions1,5,8
WARNING: Do not remove PINSYNC® from the package and do not use the adapter if the package is damaged or if the adapter comes out of the package.*
- Remove cover from the PINSYNC® package
- Place the activated DEFINITY® vial on a flat surface and hold by the base, hold the PINSYNC® package, and push the PINSYNC® vial adapter straight down onto the DEFINITY® vial top until it snaps securely in place
- Remove the package without touching the exposed end of the vial adapter as this will cause contamination
- Hold the adapter by the skirt, and attach the 10-mL syringe filled with 8.7 mL preservative-free saline to the vial adapter with a clockwise twisting motion. Do not inject air into the DEFINITY® vial
- Invert vial and slowly withdraw 1.3 mL activated DEFINITY®
- Turn vial upright, and hold vial adapter by the skirt; remove syringe from PINSYNC® with a counterclockwise twisting motion
- Gently agitate the syringe by hand to evenly distribute microbubbles
- Administer 1 to 2 mL slowly with subsequent injections as needed
*Please refer to the PINSYNC® Instructions for Use (IFU).
Diluted Bolus
DEFINITY® is the only ultrasound enhancing agent approved for use in a diluted form. Diluted bolus is the most common DEFINITY® administration option. This method simplifies administration by combining activated DEFINITY® and preservative-free saline in the same syringe.1,5
How to Prepare DEFINITY® for Diluted Bolus
Diluted Bolus Dosing With Needle Method
DEFINITY® can be administered using the traditional needle method.1
Supplies
- 18- or 20-gauge needles
- 10-mL syringe filled with 8.7 mL preservative-free saline
Instructions1,5
When using a needle method, it is important to vent the vial. This avoids negative pressure in the vial, which prevents microbubble disruption and allows for easier withdrawal.
- Vent the vial by placing needle tip in the activated DEFINITY® vial
- Attach an 18- or 20-gauge unfiltered needle to the 10-mL syringe filled with 8.7 mL preservative-free saline and place the needle in the vial
- Invert vial and slowly withdraw 1.3 mL activated DEFINITY® from the middle of the liquid without injecting air into the vial
- Gently agitate the syringe by hand to evenly distribute microbubbles
- Administer 1 to 2 mL slowly with subsequent injections as needed
For more details on the diluted bolus method, watch this instructional video.
Continuous Infusion
The DEFINITY® continuous infusion administration method combines 1.3 mL activated DEFINITY® in a 50‑mL bag of preservative-free saline, offering consistent image enhancement well suited for exams performed over an extended period. DEFINITY® is the only ultrasound enhancing agent approved for use in a diluted form.1,3,4,6,7
How to Prepare DEFINITY® for Continuous Infusion
Continuous Infusion Dosing With PINSYNC® (Vented Vial Adapter 13 mm)8,*
PINSYNC® (Vented Vial Adapter 13 mm) enables the safe, simple, and efficient transfer of activated DEFINITY® from vial to syringe.
Supplies
- PINSYNC®*
- 50-mL bag of preservative-free saline
- 3-mL syringe
- 18- or 20-gauge unfiltered needle
Instructions1,8
WARNING: Do not remove PINSYNC® from the package and do not use the adapter if the package is damaged or if the adapter comes out of the package.*
- Remove cover from the PINSYNC® package
- Place the activated DEFINITY® vial on a flat surface and hold by the base, hold the PINSYNC® package, and push the PINSYNC® vial adapter straight down onto the DEFINITY® vial top until it snaps securely in place
- Remove the package without touching the exposed end of the vial adapter as this will cause contamination
- Hold the adapter by the skirt, and attach the 3-mL syringe to the vial adapter with a clockwise twisting motion. Do not inject air into the DEFINITY® vial
- Invert vial and slowly withdraw 1.3 mL activated DEFINITY®
- Turn vial upright, and hold vial adapter by the skirt; remove syringe from PINSYNC® with a counterclockwise twisting motion
- Attach an 18- or 20-gauge unfiltered needle to the syringe and slowly add 1.3 mL activated DEFINITY® into the 50‑mL bag of preservative-free saline
- Gently agitate the bag by hand to evenly distribute microbubbles
Initiate drip rate at 4 mL/minute, titrating to a maximum of 10 mL/minute to achieve optimal image enhancement.
*Please refer to the PINSYNC® Instructions for Use (IFU).
Continuous Infusion
The DEFINITY® continuous infusion administration method combines 1.3 mL activated DEFINITY® in a 50-mL bag of preservative-free saline, offering consistent image enhancement well suited for exams performed over an extended period. DEFINITY® is the only ultrasound enhancing agent approved for use in a diluted form.1,3,4,6,7
How to Prepare DEFINITY® for Continuous Infusion
Continuous Infusion Dosing With Needle Method
DEFINITY® can be administered using the traditional needle method.1
Supplies
- 50-mL bag of preservative-free saline
- 3-mL syringe
- 18- or 20-gauge unfiltered needles
Instructions1
When using a needle method, it is important to vent the vial. This avoids negative pressure in the vial, which prevents microbubble disruption and allows for easier withdrawal.
- Vent vial with a needle by placing needle tip in the activated DEFINITY® vial
- Attach an 18- or 20-gauge unfiltered needle to the 3-mL syringe and place the needle in the vial
- Invert vial and slowly withdraw 1.3 mL activated DEFINITY® from the middle of the liquid without injecting air into the vial
- Add 1.3 mL activated DEFINITY® into the 50‑mL bag of preservative-free saline
- Gently squeeze bag to evenly distribute microbubbles
Initiate drip rate at 4 mL/minute, titrating to a maximum of 10 mL/minute to achieve optimal image enhancement.
For more details on the continuous infusion
method, watch this instructional video.
Bolus
The DEFINITY® bolus administration method is a simple, straight bolus injection, followed by a saline flush, offering easy, rapid DEFINITY® enhancement in small doses.1
How to Prepare DEFINITY® for Bolus
Bolus Dosing With PINSYNC® (Vented Vial Adapter 13 mm)8,*
PINSYNC® (Vented Vial Adapter 13 mm) enables the safe, simple, and efficient transfer of activated DEFINITY® from vial to syringe.
Supplies
- PINSYNC®*
- 10-mL syringe filled with saline
- 3-mL or tuberculin syringe
- 3-way stopcock (optional)
Instructions1,8
WARNING: Do not remove PINSYNC® from the package and do not use the adapter if the package is damaged or if the adapter comes out of the package.*
- Remove cover from the PINSYNC® package
- Place the activated DEFINITY® vial on a flat surface and hold by the base, hold the PINSYNC® package, and push the PINSYNC® vial adapter straight down onto the DEFINITY® vial top until it snaps securely in place
- Remove the package without touching the exposed end of the vial adapter as this will cause contamination
- Hold the adapter by the skirt, and attach the 3-mL or tuberculin syringe to the vial adapter with a clockwise twist and without injecting air into the DEFINITY® vial
- Invert vial and slowly withdraw 10 µL/kg activated DEFINITY®
- Turn vial upright, and hold vial adapter by the skirt; remove syringe from PINSYNC® with a counterclockwise twisting motion
- Administer slowly over 30 to 60 seconds. Follow with a 10-mL saline flush. Administer a subsequent injection as needed 30 minutes after the first injection, followed by saline flush
Smaller, incremental dose amounts of 0.1 to 0.2 mL are better suited for current ultrasound system technology.7
Maximum allowable dose is 2 bolus doses.1
*Please refer to the PINSYNC® Instructions for Use (IFU).
Bolus
The DEFINITY® bolus administration method is a simple, straight bolus injection, followed by a saline flush, offering easy, rapid DEFINITY® enhancement in small doses.1
How to Prepare DEFINITY® for Bolus
Bolus Dosing With Needle Method
DEFINITY® can be administered using the traditional needle method.1
Supplies
- 18- or 20-gauge unfiltered needles
- 10-mL syringe filled with saline
- 3-mL or tuberculin syringe
- 3-way stopcock (optional)
Instructions1
When using a needle method, it is important to vent the vial. This avoids negative pressure in the vial, which prevents microbubble disruption and allows for easier withdrawal.
- Vent vial with a needle by placing needle tip in the activated DEFINITY® vial
- Attach an 18- or 20-gauge unfiltered needle to the 3‑mL or tuberculin syringe and place needle in the vial
- Invert vial and slowly withdraw 10 µL/kg activated DEFINITY® from the middle of the liquid without injecting air into the vial
- Administer slowly over 30 to 60 seconds. Follow with a 10‑mL saline flush. Administer a subsequent injection as needed 30 minutes after the first injection, followed by saline flush
Smaller, incremental dose amounts of 0.1 to 0.2 mL are better suited for current ultrasound system technology.7
Maximum allowable dose is 2 bolus doses.1
For more details on the bolus method,
watch this instructional video.
Bolus Pediatric
The DEFINITY® bolus administration method is a simple, straight bolus injection, followed by a saline flush, offering easy, rapid DEFINITY® enhancement in small doses.1
How to Prepare DEFINITY® for Bolus
Bolus Dosing With PINSYNC® (Vented Vial Adapter 13 mm)8,*
PINSYNC® (Vented Vial Adapter 13 mm) enables the safe, simple, and efficient transfer of activated DEFINITY® from vial to syringe.
Supplies
- PINSYNC®*
- 5-mL syringe filled with saline
- 3-mL or tuberculin syringe
- 3-way stopcock (optional)
Instructions1,8
WARNING: Do not remove PINSYNC® from the package and do not use the adapter if the package is damaged or if the adapter comes out of the package.*
- Remove cover from the PINSYNC® package
- Place the activated DEFINITY® vial on a flat surface and hold by the base, hold the PINSYNC® package, and push the PINSYNC® vial adapter straight down onto the DEFINITY® vial top until it snaps securely in place
- Remove the package without touching the exposed end of the vial adapter as this will cause contamination
- Hold the adapter by the skirt, and attach the 3-mL or tuberculin syringe to the vial adapter with a clockwise twist and without injecting air into the DEFINITY® vial
- Invert vial and slowly withdraw 3 µL/kg activated DEFINITY®
- Turn vial upright, and hold vial adapter by the skirt; remove syringe from PINSYNC® with a counterclockwise twisting motion
- Administer slowly over 30 to 60 seconds. Follow with a 5-mL saline flush. Administer a subsequent 3 to 5 µL/kg injection as needed 30 minutes after the first injection, followed by a saline flush
Maximum allowable dose for pediatric patients is 2 bolus doses, 30 minutes apart.1
*Please refer to the PINSYNC® Instructions for Use (IFU).
Bolus Pediatric
The DEFINITY® bolus administration method is a simple, straight bolus injection, followed by a saline flush, offering easy, rapid DEFINITY® enhancement in small doses.1
How to Prepare DEFINITY® for Bolus
Bolus Dosing With Needle Method
DEFINITY® can be administered using the traditional needle method.1
Supplies
- 18- or 20-gauge unfiltered needles
- Syringe filled with 5 mL saline
- 3-mL or tuberculin syringe
- 3-way stopcock (optional)
Instructions1
When using a needle method, it is important to vent the vial. This avoids negative pressure in the vial, which prevents microbubble disruption and allows for easier withdrawal.
- Vent vial with a needle by placing needle tip in the activated DEFINITY® vial
- Attach an 18- or 20-gauge unfiltered needle to the 3‑mL or tuberculin syringe and place needle in the vial
- Invert vial and slowly withdraw 3 µL/kg activated DEFINITY® from the middle of the liquid without injecting air into the vial
- Administer slowly over 30 to 60 seconds. Follow with a 5‑mL saline flush. Administer a subsequent 3 to 5 μL/kg injection as needed 30 minutes after the first injection, followed by a saline flush
Maximum allowable dose for pediatric patients is 2 bolus doses, 30 minutes apart.1
An echocardiogram was performed to evaluate left ventricular wall motion, status post an acute myocardial infarction
Conclusion
- The patient was started on systemic anticoagulation with intravenous heparin and medical therapy was initiated for congestive heart failure
- Neurologic symptoms with the presence of a left ventricular thrombus confirmed the need for a brain MRI for assessment of stroke
- Cardiomyopathy confirmed the need for ischemic evaluation
- Surgical consultation was initiated for possible thrombolytic therapy vs surgical embolectomy
DEFINITY® in the critically ill enhanced overall efficiency, diagnostic accuracy, and cost-effective patient management1
Have a DEFINITY®
case study to share?
CK-MB=creatine kinase muscle and brain; MRI=magnetic resonance imaging.
An echocardiogram was performed to evaluate left ventricular wall motion, status post an acute myocardial infarction
Apical 4-chamber
Unenhanced
Enhanced with DEFINITY®
Apical 2-chamber
Unenhanced Echo
- Hypokinetic left ventricular segments with suspected thrombus in the apex with a high concern for embolic risk
DEFINITY® Echo
- Hypokinetic left ventricular segments with severely decreased systolic function
- A large, bulbous, solid, partially fixed thrombus in the apex of the scarred, thinned, and akinetic left ventricle was identified
- A well-defined space was confirmed by the microbubbles separating a large area of thrombus from the apex, revealing a very high risk of embolism
See the inside story of your patients
Have a DEFINITY®
case study to share?
CK-MB=creatine kinase muscle and brain; MRI=magnetic resonance imaging.
References:
-
DEFINITY® [package insert]. N. Billerica, MA: Lantheus Medical Imaging, Inc.
-
VialMix® User's Guide. N. Billerica, MA: Lantheus Medical Imaging, Inc.
-
Optison™ [package insert]. Marlborough, MA: GE Healthcare Inc.
-
Lumason® [package insert]. Monroe Township, NJ: Bracco Diagnostics Inc.
-
Data on File, N. Billerica, MA: Lantheus Medical Imaging, Inc.
-
PINSYNC. Instructions for use. Lantheus Medical Imaging, Inc; 2018.
INDICATION
DEFINITY® is indicated, after activation, for use in adult and pediatric patients with suboptimal echocardiograms to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following perflutren-containing microsphere administration. Most serious reactions occur within 30 minutes of administration.
- Assess all patients for the presence of any condition that precludes DEFINITY® administration.
- Always have resuscitation equipment and trained personnel readily available.
References:
- DEFINITY®. Prescribing Information. Lantheus.
- VIALMIX®. User's Guide. Lantheus Medical Imaging, Inc; 2018.
- Lumason®. Package insert. Bracco Diagnostics Inc; 2023.
- Optison™. Package insert. GE Healthcare Inc; 2021.
- Data on file, Lantheus.
- Lindner JR. A practical approach to contrast echocardiography. American College of Cardiology. July 10, 2017. Accessed March 18, 2021. https://www.acc.org/latest-in-cardiology/articles/2017/07/10/09/17/a-practical-approach-to-contrast-echocardiography
- Porter TR, Mulvagh SL, Abdelmoneim SS, et al. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography guidelines update. J Am Soc Echocardiogr. 2018;31(3):241-274. doi:10.1016/j.echo.2017.11.013
- PINSYNC. Instructions for use. Lantheus Medical Imaging, Inc; 2018.
DEFINITY® Customer Service 1.800.299.3431
 BACK TO
BACK TO TOP
